A homogeneous luminescent immunoassay detection technology that generates light as the result of the interaction of HRP and acridan in the presence of hydrogen peroxide was evaluated in the validation of a pharmacokinetic (PK) assay for human IgG drug in rat serum. A commercially available SPARCL kit was purchased and an automated robotic pipetting station was used for robotic pipetting station provided the liquid handling in the execution of the SPARCL commercial kit and was investigated for labor hours required, and assay performance including; dynamic range, minimum required dilution, precision, accuracy and total error.
Sparcl™ technology
SPARCLTM (Spatial Proximity Analyte Reagent Capture Luminescence) technology is a proximity dependent chemiluminescent detection method. In a SPARCL assay, a chemiluminescent compound (acridan) is brought into close proximity to an oxidative enzyme (horseradish peroxidase, HRP) through specific antigen/antibody interaction. A flash of light proportional to the quantity of analyte present in the sample is generated upon addition of a trigger solution containing hydrogen peroxide. A background reducing agent (BGR) is often used to enhance S/N ratios. There is no need to remove excess reactants. There are no washing steps.
Specific antibody and antigen interaction brings acridan and HRP into close proximity. The addition of hydrogen peroxide based trigger solution interacts with acridan and HRP, causing a flash of light.
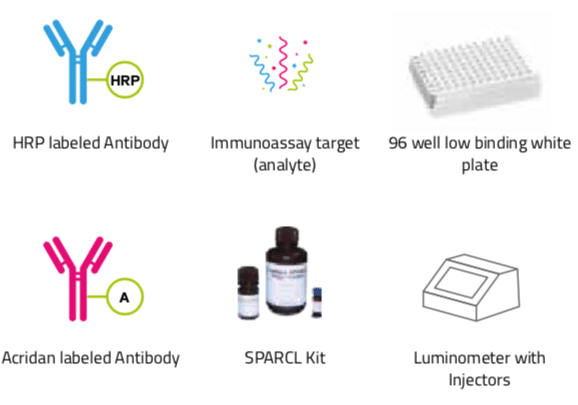
Figure 1: Sparcl key components
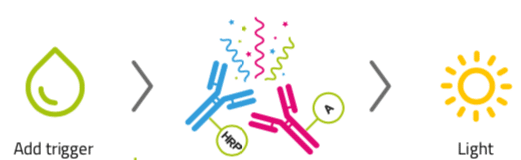
Figure 2: A representative Sparcl assay
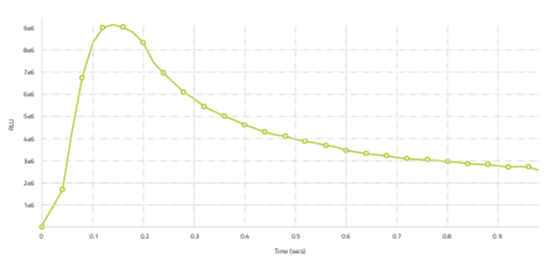
Figure 3: Typical Sparcl luminescence profile
Note that the time is on the X-axis and is from zero to 1 second. Relative light units (RLU) is on the Y-axis. There is a characteristic spike in light at 0.15 seconds after addition of trigger solution and predictable decay following the spike.
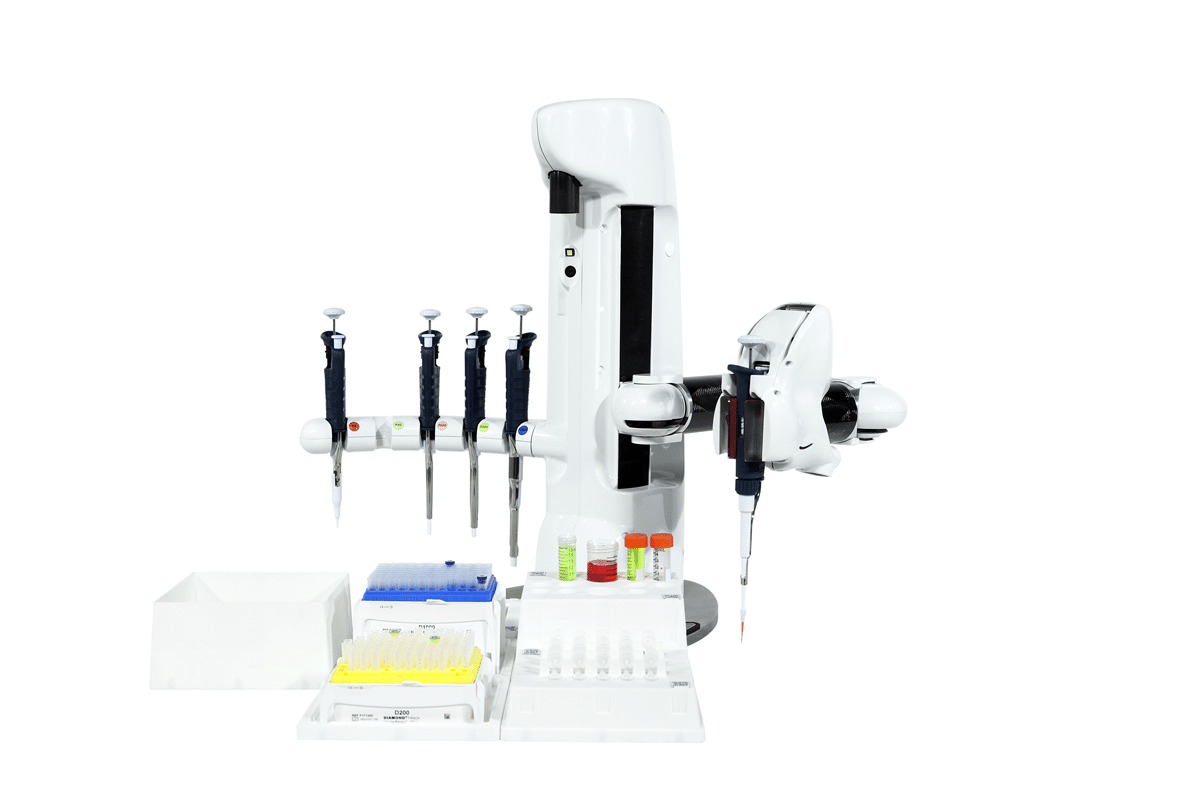
Figure 4: The Andrew pipetting station
Andrew uses commonly used laboratory pipets and pipet tips to perform all the liquid handling steps of the SPARCL immunoassay method.
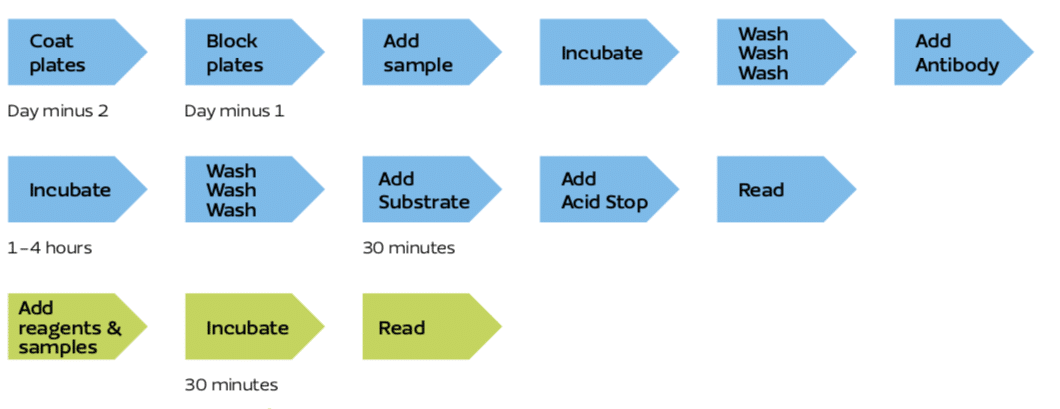
Figure 5: Typical ELISA workflow in blue and typical SPARCL workflow in green.
SPARCL does not use antibody coated plates nor does SPARCL have any washing steps.
Materials and methods
SPARCL PK Kit: Catalog number IGG-SP-20, Life Diagnostics, Inc. (West Chester, Pennsylvania).
Humira: Also known as Adalimumab, is a fully human recombinant immunoglobulin, is IgG1 and is an anti-TNF monoclonal antibody. It was obtained from the local pharmacy.
Andrew 1000G liquid handling robot: Andrew Alliance (Boston, Massachusetts).
Pipets: Gilson, Pipetman (Middleton, Wisconsin).
Matrix: Rat serum. Bioreclamation (Baltimore, Maryland). Used at 10% in assay buffer. Assay buffer provided by Life Diagnostics. Luminometer: BMG LabTech’s LUMIstar Omega.
The SPARCL PK kit was run according to the directions provided by the manufacturer (Life Diagnostics) by giving the pipetting instructions to the Andrew robot. Briefly, Andrew made the dilutions for the standard curve and QC’s, pipetted the antibodies, calibrators and QC’s into the wells of the 96 well plates.
The 96 well plate was incubated at room temperature for 30 minutes on a orbital shaker set at 350 revolutions per minute. After the incubation time was complete, the plate was moved to the LUMIstar Omega luminometer. The LUMIstar was instructed to inject the trigger solution and collect the emitted light.
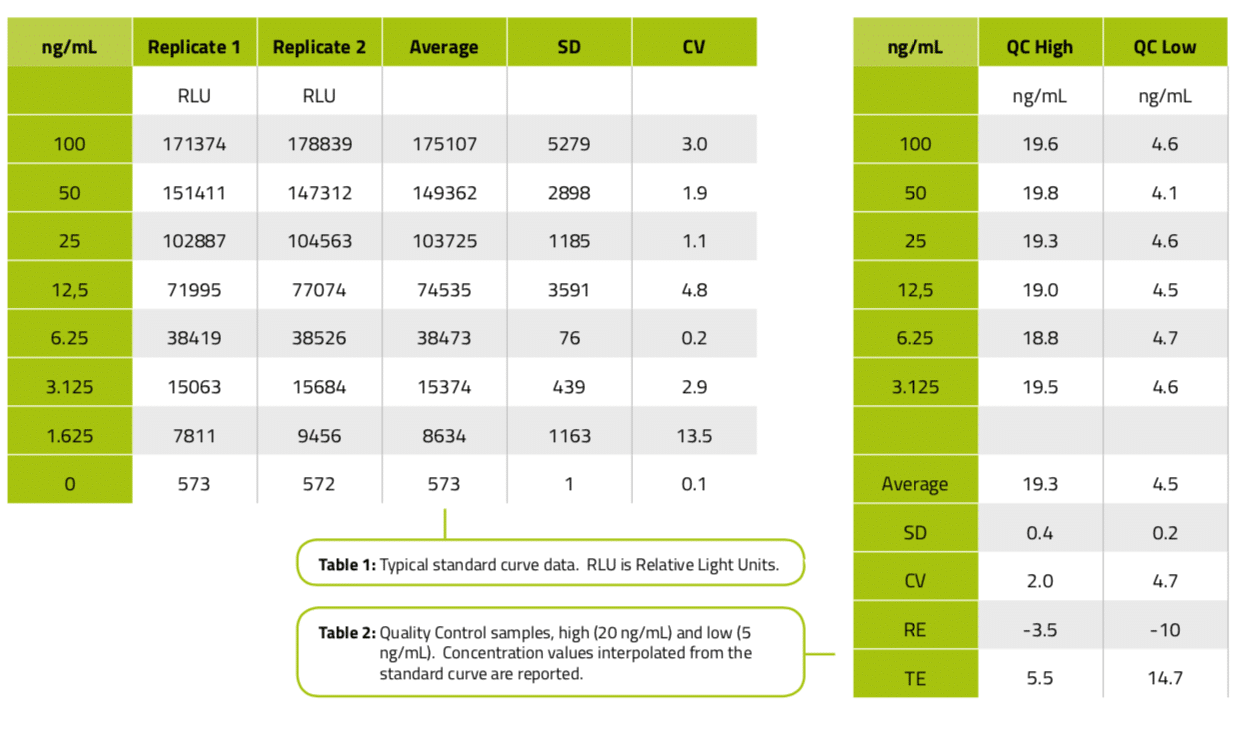
Conclusions and future direction
The use of the Andrew robot with the Life Diagnostics SPARCL PK kit provides an assay that is accurate and precise for Humira in rat matrix.
The Andrew robot and the Life Diagnostics SPARCL PK kit reduces hu- man input and error, offers improvement in workflow and efficiencies.
The Andrew system offers compliant software which may make the system useful in regulated laboratories.
The combination of the Andrew robot with the Life Diagnostics SPARCL kit may be a solution for labs measuring IgG drug concentration in a preclinical setting.
The combination of the Andrew robot with the Life Diagnostics SPARCL PK kit may be a way for CRO’s to improve efficiencies, reduce PK assay development time and cost, reduce labor and increase profitability.
